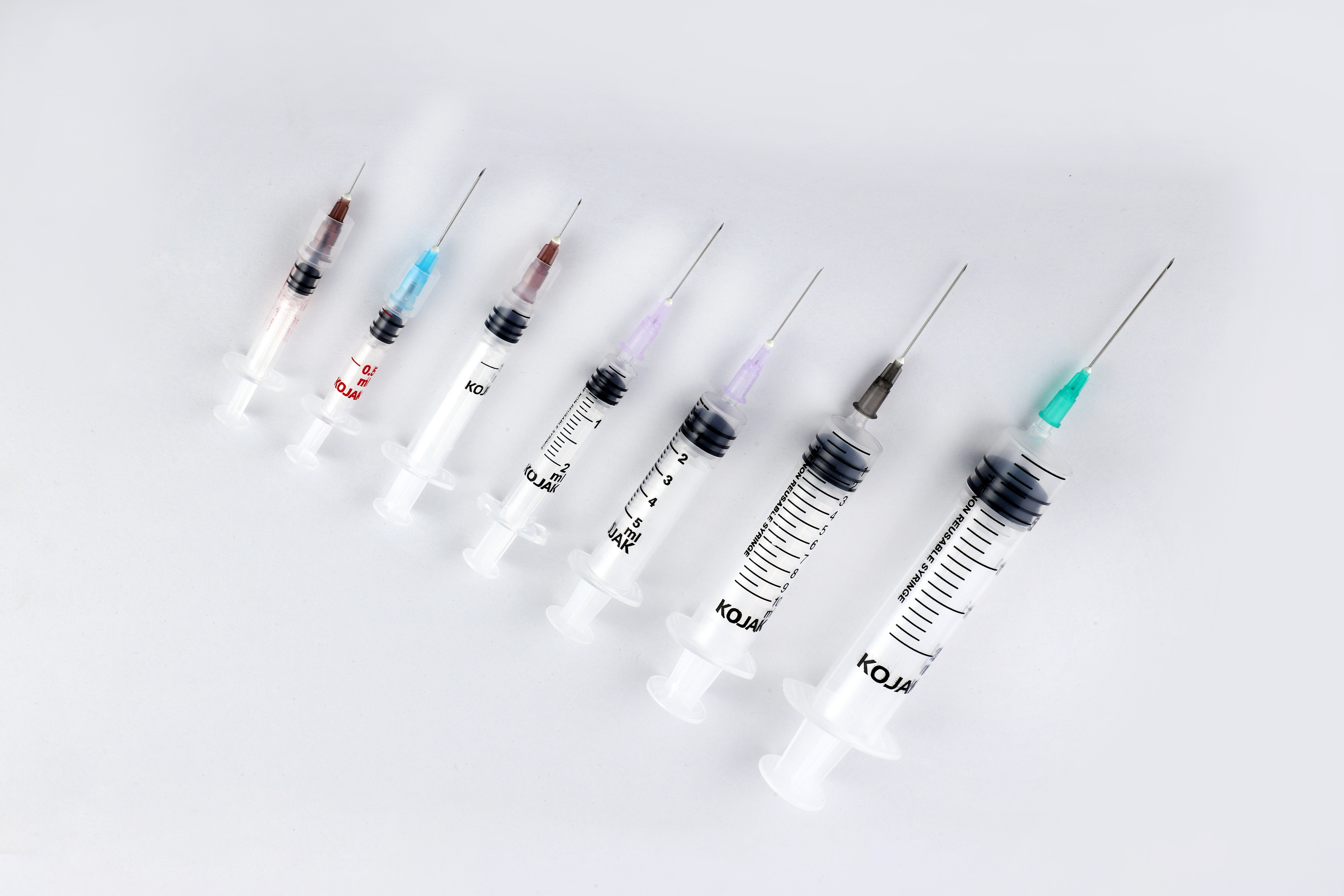


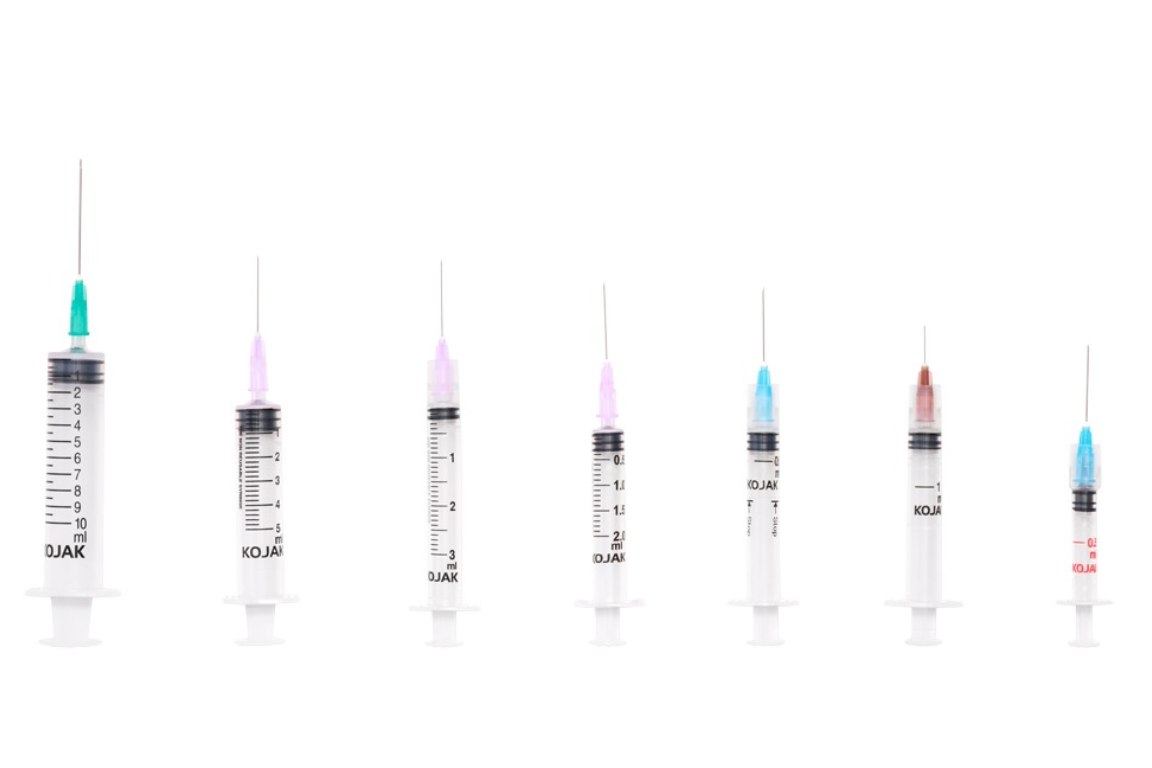

THE AD SYRINGE COMES WITH THE FOLLOWING ADVANTAGES:
- It gets automatically blocked after injection, ruling out chances of reuse and thereby preventing the transmission of pathogens between patients.
- It is ideal for use in areas where disposal systems are inadequate and reuse of disposable syringes is common.
- Their cost is very low as compared to the cost of treating diseases transmitted via infected syringes and needles. Thus, they are the most effective and affordable way to prevent illnesses.



THE KOJAK AUTO-DISABLE HYPODERMIC SYRINGE
HMD Ltd. introduced the AD syringe in India in 2001, becoming the first auto disable syringe manufacturers in the country. It was launched in technical collaboration with United Kingdom-based global licensing company Star Syringes, which had patented the design. Sold under the brand name Kojak Selinge. It is available in the curative healthcare segment for various usage – in 2 ml, 3 ml, 5 ml, 10 ml, and 20 ml sizes. For immunization, it is available in 0.1 ml, 0.3ml, 0.5 ml, and 1 ml sizes. Kojak Selinge works on a ring & break mechanism which makes sure that the plunger breaks if re-use is attempted. An affordably priced syringe, it is available with any gauge of needle that is preferred by the user. Both detachable and fixed needle design options are present. Additional safety is ensured through the Luer-Lok tip – which enables the needle to be locked in place – and Luer slip tip – which enables a quicker fit. Auto-disable syringes are playing a major role in preventing and combating the spread of COVID-19, the deadly pandemic that has gripped the world since 2020. From the very beginning, WHO suggested the use of auto-disable syringes for collecting blood samples of Coronavirus patients, in order that the disease is not transmitted via healthcare equipment. Further, being recommended for giving vaccines in public immunization programmes, it is also playing a critical role in administrating the COVID-19 vaccine. The largest manufacturer of auto-disable syringes in the world, HMD Ltd. plans to scale up its production capacity from 700 million AD Syringes a year to one billion by mid-2021 as part of its commitment to produce an adequate number of AD syringes for the COVID-19 vaccination effort. One of the largest suppliers of auto-disable syringes to UNICEF for its immunization programmes for more than a decade, HMD has received orders for multiple syringes from UNICEF for the COVAX facility - a global initiative meant to accelerate the development and manufacture of COVID-19 vaccines, and to ensure that poorer nations have equitable access to it. At least 60 to 70 percent of the 1.3 billion people in India and 7.8 billion people in the world will need auto-disable syringes for the COVID-19 vaccine. Accordingly, we at HMD plan to allocate 50 percent of the total 0.3ML and 0.5 ML AD syringes for India and 50 percent for export to other countries, in keeping with our vision to contribute to affordable access and patient safety everywhere.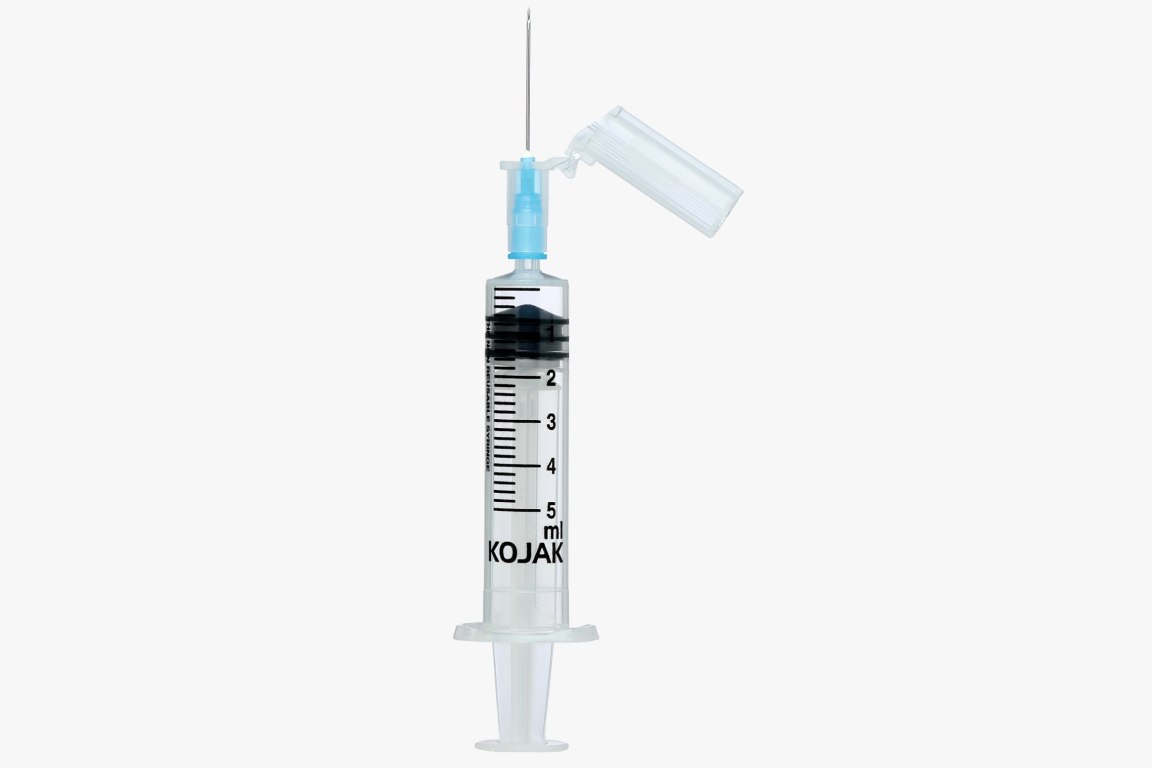

KOJAK SMART SYRINGE
- Equipped with a Safety SIP (Sharp Injury Prevention) Shield, with Auto disable feature not only safeguarding healthcare workers from accidental needle stick injuries (NSI’s) but also preventing syringe reuse.
- K1 design, equipped with a unique ring and lock mechanism, where the plunger breaks automatically, if reuse is attempted. Thus mitigating the risk of cross-contamination while reducing unsafe infection practices.
- Aspiration can be easily performed and can also be used for reconstitution of medication
- Available for both Curative and Immunization uses
- Added safety with SIP shield feature, which covers the needle after the injection procedure by gently pressing the needle cover on hard surface, thereby safeguarding the healthcare workers from needle stick injuries.
FAQ's
Auto Disable Syringe are Syringe that cannot be reused. They incorporate a mechanism to break or lock the plunger when the injection is given to make the syringe inoperable for being used for second time.
HMD has been educating Public Healthcare Officers of the need for injection safety via AD Syringes. Field staff of HMD have conducted CME Programmes w.r.t. the usage of Auto disable syringes & importance of AD Syringes for curative purpose in almost all the leading hospitals in India. As far as
govt. sector is concerned, HMD has regularly been participating in AD Syringes tenders of State Govt. HMD has been sending communication to the various state Govt. Officials, emphasizing on importance of AD Syringes for curative sector.
Karnataka, MP, Delhi, North East states, Orissa, Kerala, Maharashtra, West Bengal, Jharkhand have been more progressive and receptive to modernize.
-
Do not push the plunger forward to avoid activating the Reuse mechanism.
-
Draw back the plunger only once.
-
It’s not necessary to aspirate when injecting EPI Vaccines.
As AD Syringe is primary targeted for public healthcare consumption initially, the growth is dependent upon Government policies. If the Government is smart and willing to invest for the long term benefit in preventive healthcare to reduce noscomial infections they will see the benefits of this technology. Radial tyres are more expensive than Nylon Tyres. They however last much longer so smart consumer buy them as they are more cost effective.
if Government wants to reduce the ALS (Average Length of Stay) of patients in hospitals and save money – AD Syringes is good starting point to bring about change as SPI (Safer Patient Initiative)
Auto Disable syringe is a syringe that cannot be reused. It incorporates a mechanism to automatically break or lock the plunger as the injection is being given to make the syringe inoperable for possibly being used for a second time
-
Exploding the myth of high prices and unaffordability.
-
Exploding the myth of lack of adequate availability and capacity
-
Exploding the myth of ‘Hospital Waste Management’ as a substitute for ‘AD Syringes Deployment’ for ensuring injection safety through non reuse.
-
Denial of the problem of Reuse of Syringes in public and private healthcare institutes and of unsafe injection practices
AD Syringes were used in immunization projects by UNICEF/PATH even prior to 2000. For curative injections HMD introduced AD Syringes first time in India in the year 2002
Auto Disable Syringe cannot be reused.